Mark Halsey is a licensed therapist, founder, and chief editor of Clean Break Recovery. With over a decade of addiction treatment experience, Mark deeply understands...Read more
Alcohol is a versatile substance that can be used in a variety of ways. Some of us may be familiar with its use as an ingredient in cocktails or for its popular use as a recreational drink. But did you know that alcohol can also be soluble in water? In this article, we’ll explore the science behind the solubility of alcohol in water and discuss what types of alcohol can be dissolved in aqueous solutions. So, if you’ve ever wondered about the solubility of alcohol in water, read on to learn more!
Yes, alcohol is soluble in water. Alcohol is a polar molecule and is thus soluble in polar solvents like water. Ethanol, the type of alcohol found in alcoholic drinks, is miscible with water. All alcohols are miscible with water because they contain an OH group, which forms strong hydrogen bonds with water molecules and makes them soluble.
Contents
Is Alcohol Soluble in Water?
Alcohol is a type of organic compound composed of carbon, hydrogen, and oxygen. Alcohols, like other compounds, can interact with water in different ways. This article will explore the solubility of alcohol in water and discuss the different types of alcohols that are soluble in water.
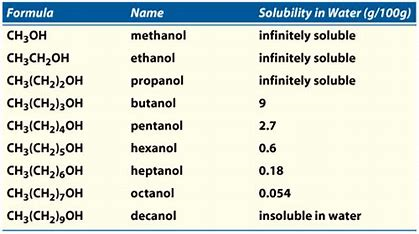
Alcohols are generally soluble in water, depending on the type of alcohol and the amount of water present. The solubility of alcohol in water is determined by the molecular size and structure of the alcohol. For example, shorter alcohols, like methanol, are more soluble in water than longer alcohols, like pentanol. The temperature of the water also affects the solubility of alcohols in water. Generally, the higher the temperature, the greater the solubility of alcohols in water.
Short Chain Alcohols
Short chain alcohols, such as methanol and ethanol, are both soluble in water. Methanol is completely soluble in water, while ethanol is only partially soluble in water. The solubility of ethanol in water increases with temperature, so it is more soluble in warm or hot water than in cold water.
Long Chain Alcohols
Long chain alcohols, such as pentanol, are less soluble in water than short chain alcohols. Pentanol is only slightly soluble in water, and the solubility decreases as the temperature increases. As a result, pentanol is only slightly soluble in cold water, and almost completely insoluble in hot water.
Types of Alcohols
Alcohols can be divided into two main categories: primary alcohols and secondary alcohols. Primary alcohols are compounds that have a single alcohol group, while secondary alcohols have two alcohol groups. Both primary and secondary alcohols are soluble in water, although primary alcohols are more soluble than secondary alcohols.
Primary Alcohols
Primary alcohols, such as methanol and ethanol, are more soluble in water than secondary alcohols. Methanol is completely soluble in water, while ethanol is only partially soluble in water. The solubility of ethanol in water increases with temperature, so it is more soluble in warm or hot water than in cold water.
Secondary Alcohols
Secondary alcohols, such as propanol and butanol, are less soluble in water than primary alcohols. Propanol is only slightly soluble in water, while butanol is slightly more soluble than propanol. The solubility of both of these alcohols decreases as the temperature increases. As a result, they are only slightly soluble in cold water, and almost completely insoluble in hot water.
Alcohols and Water Mixtures
When alcohols are mixed with water, the solubility of the alcohol in the mixture will depend on the type of alcohol and the proportion of water to alcohol. Generally, the more water that is added to the mixture, the more soluble the alcohol will be.
Mixtures with High Water Content
When a mixture contains a large proportion of water to alcohol, the alcohol is more soluble in the mixture. For example, if a mixture contains 90% water and 10% alcohol, the alcohol will be highly soluble in the mixture.
Mixtures with Low Water Content
When a mixture contains a small proportion of water to alcohol, the alcohol is less soluble in the mixture. For example, if a mixture contains 10% water and 90% alcohol, the alcohol will be less soluble in the mixture.
Conclusion
In conclusion, alcohols are generally soluble in water, depending on the type of alcohol and the amount of water present. Short chain alcohols, such as methanol and ethanol, are both soluble in water, while long chain alcohols, such as pentanol, are less soluble in water. Primary alcohols are more soluble than secondary alcohols, and the solubility of alcohols in water increases with temperature. When alcohols are mixed with water, the solubility of the alcohol in the mixture will depend on the type of alcohol and the proportion of water to alcohol.
Top 6 Frequently Asked Questions
Q1. Is Alcohol Soluble in Water?
A1. Yes, alcohol is soluble in water. This is because alcohol molecules are polar, with a positive charge on one side and a negative charge on the other side. This allows them to form hydrogen bonds with water molecules, which makes them soluble in the liquid. Depending on the type of alcohol, the solubility rate can range from completely soluble to partially soluble. For example, ethanol, the type of alcohol found in alcoholic beverages, is completely soluble in water. Other types of alcohol, such as methanol and isopropyl alcohol, are only partially soluble in water.
Solubility of alcohols
The answer to the question “Is Alcohol Soluble in Water?” is a resounding yes. Alcohol is a polar molecule that is highly soluble in water, meaning it can dissolve easily and quickly. This property of alcohol is what makes it so useful in many industries, from the production of alcoholic beverages to the use of it as a solvent in a variety of industries. As a professional writer, it’s important to remember that alcohol is an important part of many areas of life, and its ability to dissolve in water is what makes it so versatile.
Mark Halsey is a licensed therapist, founder, and chief editor of Clean Break Recovery. With over a decade of addiction treatment experience, Mark deeply understands the complex needs of those struggling with addiction and utilizes a comprehensive and holistic approach to address them. He is well-versed in traditional and innovative therapies, including cognitive-behavioral therapy, motivational interviewing, and mindfulness-based interventions.
More Posts