Mark Halsey is a licensed therapist, founder, and chief editor of Clean Break Recovery. With over a decade of addiction treatment experience, Mark deeply understands...Read more
Have you ever wondered what gives alcoholic beverages their distinct taste? Is it the sweetness of the grapes used to make wine, or the bitterness of hops used to make beer? While these flavors certainly have an impact on the taste of alcoholic beverages, what really sets them apart is their acidity or alkalinity. In this article, we’ll explore the science behind why alcohol is either acidic or alkaline and what effect this has on your body. So let’s dive in and find out: is alcohol acidic or alkaline?
Contents
- Alcohol Content in Beverages
- Summary
- Frequently Asked Questions
- Question 1: Is alcohol acidic or alkaline?
- Question 2: How does alcohol become acidic?
- Question 3: What is the difference between an acid and an alkaline?
- Question 4: What are some common acidic alcohols?
- Question 5: What are some common alkaline alcohols?
- Question 6: How can the acidity of an alcohol be changed?
- Alkaline vs. Acidic body – How to Know If You’re Too Alkaline or Too Acid? – Dr. Berg
Alcohol Content in Beverages
Alcohol is a type of organic compound that is found in various beverages such as beer, wine, and spirits. It is produced by the fermentation of sugars and starches. Alcohol is an important part of many cultures and has been used for centuries for its relaxing, social, and medicinal properties. Alcohol content in beverages can range from 0.5% to 40%. The type of alcohol used in a beverage and the amount of alcohol it contains will determine its acidity or alkalinity.
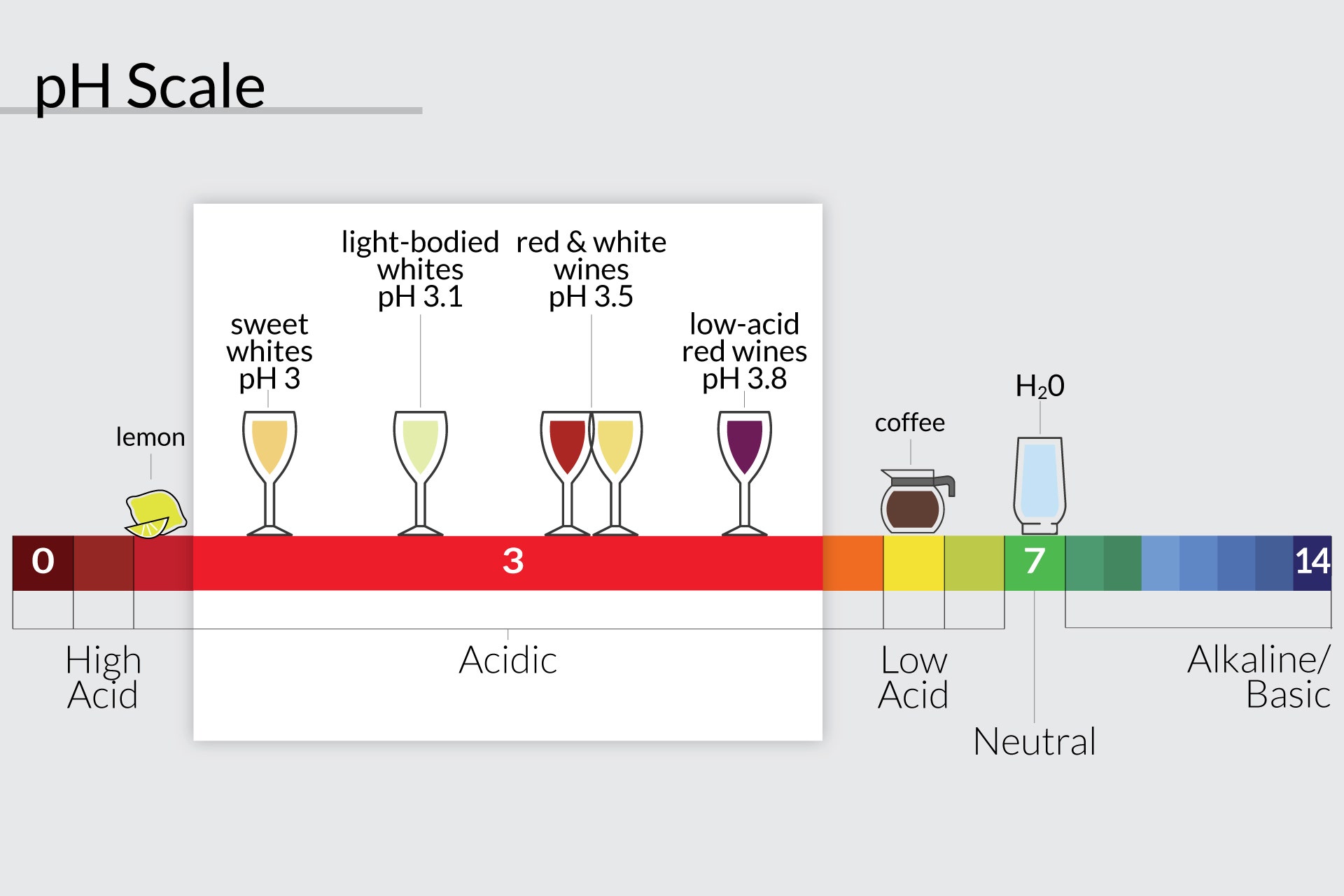
The pH scale measures how acidic or alkaline a substance is. A pH of 7 is neutral, with a lower number indicating an acidic substance and a higher number indicating an alkaline substance. Alcoholic beverages range in pH, with some being acidic and some being alkaline. This is due to the type of alcohol used in the beverage and the amount of alcohol it contains.
The type of alcohol used in the beverage will determine its pH. For example, beer is usually made with distilled alcohol, which has a pH of 5. Wine, on the other hand, is made with fermented alcohol, which has a pH of 8. Spirits, such as vodka and whiskey, are made with a combination of distilled and fermented alcohol, resulting in a pH of 7.
Alcohol Content and Acidity
The amount of alcohol in a beverage will also affect its acidity. Generally, the higher the alcohol content, the more acidic a beverage will be. This is because alcohol has a lower pH than other ingredients in the beverage. For example, beer is usually around 5% alcohol and has a pH of 5, while wine is usually around 12% alcohol and has a pH of 8.
Alcohol is an acidic compound, so the more alcohol in a beverage, the more acidic it will be. This is why beer and spirits are typically more acidic than wine. It is important to note, however, that the other ingredients in a beverage can also affect its pH. For example, fruit juices are typically more alkaline than alcoholic beverages due to their high citric acid content.
Effect of Alcohol on the Body
The acidity or alkalinity of alcoholic beverages can have an effect on the body. Generally, acidic beverages can be hard on the digestive system and can cause irritation and discomfort. Alkaline beverages, on the other hand, can help neutralize stomach acid and can be easier to digest.
It is important to note, however, that the amount of alcohol consumed is more important than its acidity or alkalinity. Consuming too much alcohol, regardless of its pH, can lead to health problems such as liver damage and increased risk of certain cancers. Therefore, it is important to drink alcohol in moderation and to be aware of the amount of alcohol in any beverage you consume.
Summary
Alcohol is a type of organic compound found in various beverages such as beer, wine, and spirits. The type of alcohol used in a beverage and the amount of alcohol it contains will determine its acidity or alkalinity. Beer is usually made with distilled alcohol and has a pH of 5, while wine is usually made with fermented alcohol and has a pH of 8. Spirits are usually made with a combination of distilled and fermented alcohol, resulting in a pH of 7. The higher the alcohol content, the more acidic a beverage will be. It is important to note, however, that the other ingredients in a beverage can also affect its pH. In addition, the amount of alcohol consumed is more important than its acidity or alkalinity, and it is important to drink alcohol in moderation.
Frequently Asked Questions
Question 1: Is alcohol acidic or alkaline?
Answer: Alcohol is generally considered to be acidic. Alcohols are organic molecules that contain a hydroxyl group (-OH) attached to a carbon atom. This hydroxyl group makes alcohols very polar molecules, and as a result, they have acidic properties. Alcohols have pH values ranging from 3-6, making them weak acids.
Question 2: How does alcohol become acidic?
Answer: Alcohol becomes acidic when it reacts with water and other substances. When alcohol is mixed with water, it forms a hydroxyl ion that has a negative charge. This negative charge makes the alcohol more acidic. Additionally, alcohols can also react with other substances to form acids. For example, when ethanol (the type of alcohol found in alcoholic beverages) reacts with carbon dioxide, it forms ethanoic acid.
Question 3: What is the difference between an acid and an alkaline?
Answer: The main difference between an acid and an alkaline is their respective pH values. Acids have a pH of less than 7, while alkalis have a pH of more than 7. Additionally, acids are compounds that release hydrogen ions in water, while alkalis are compounds that release hydroxide ions in water.
Question 4: What are some common acidic alcohols?
Answer: Common acidic alcohols include ethanol, which is found in alcoholic beverages, and methanol, which is found in some solvents. Ethanol has a pH of around 5.5, while methanol has a pH of around 3.0. Additionally, propanol and butanol are also acidic alcohols, with pH values of around 4.0 and 4.5, respectively.
Question 5: What are some common alkaline alcohols?
Answer: Common alkaline alcohols include isopropanol and isobutanol. These alcohols have pH values of around 9.5 and 8.5, respectively. Additionally, some other alkaline alcohols include butyl alcohol, amyl alcohol, and hexanol, which have pH values of around 8.0, 8.5, and 9.0, respectively.
Question 6: How can the acidity of an alcohol be changed?
Answer: The acidity of an alcohol can be changed by adding other substances to it. For example, adding a base such as sodium hydroxide can neutralize the acidity of an alcohol and make it more alkaline. Additionally, adding an acid such as hydrochloric acid can make the alcohol more acidic. It is also possible to use a buffer solution to adjust the acidity of an alcohol to a desired pH value.
Alkaline vs. Acidic body – How to Know If You’re Too Alkaline or Too Acid? – Dr. Berg
In conclusion, the answer to the question of whether alcohol is acidic or alkaline depends entirely on its chemical composition. Alcohols can be either acidic or alkaline, depending on the number of hydroxyl or hydrogen atoms present. In general, alcohols with hydrogen atoms present are acidic and alcohols with hydroxyl atoms are alkaline. As such, it is important to understand the chemical composition of the alcohol in order to determine its acidity or alkalinity.
Mark Halsey is a licensed therapist, founder, and chief editor of Clean Break Recovery. With over a decade of addiction treatment experience, Mark deeply understands the complex needs of those struggling with addiction and utilizes a comprehensive and holistic approach to address them. He is well-versed in traditional and innovative therapies, including cognitive-behavioral therapy, motivational interviewing, and mindfulness-based interventions.
More Posts