Mark Halsey is a licensed therapist, founder, and chief editor of Clean Break Recovery. With over a decade of addiction treatment experience, Mark deeply understands...Read more
Alcohol has often been seen as an intoxicating and sometimes dangerous beverage, but did you know it can also be used to conduct electricity? In this article, we will explore the science behind how alcohol conducts electricity and why it has the ability to do so. We will also discuss the potential risks and benefits of using alcohol as a conductor. So whether you are a scientist, engineer, or just curious, read on to learn more about this unusual property of alcohol.
Contents
- Does Alcohol Possess the Ability to Conduct Electricity?
- Does Alcohol Conduct Electricity?
- Related Faq
- Does Alcohol Conduct Electricity?
- What Are the Properties of Alcohol That Make it Non-Conductive?
- What Are Some Examples of Substances That Do Conduct Electricity?
- What Are Some Uses of Alcohol That Do Not Require It to Conduct Electricity?
- What Are Some Safety Precautions to Take When Handling Alcohol?
- Are There Any Alternatives to Alcohol for Conducting Electricity?
- Do alcohol conducts electricity | Semi conductor of electricity | #shorts #ArtandExperiment
Does Alcohol Possess the Ability to Conduct Electricity?
The ability of a substance to conduct electricity is determined by its electrical properties. In this article, we will explore the electrical properties of alcohol and answer the question: Does alcohol conduct electricity?
Alcohols are organic compounds that contain a hydroxyl group (-OH) attached to a carbon atom. The electrical properties of alcohol depend on the type of alcohol and its molecular structure. Generally, alcohols possess a low electrical conductivity, but some alcohols can conduct electricity.
Alcohols are polar molecules, meaning that they have a positive and negative end. This allows them to form hydrogen bonds with other polar molecules, which makes them more likely to conduct electricity.
Methanol
Methanol is a type of alcohol that can conduct electricity. It is a clear, colorless liquid with a boiling point of 64.7 °C (148.5 °F). Methanol has a relatively low electrical conductivity, but it is still able to conduct electricity. This is due to the fact that methanol is a polar molecule and is able to form hydrogen bonds with other polar molecules.
Methanol is also a fuel, and it is used in some types of internal combustion engines. Methanol is also used as an antifreeze and as a solvent in various industrial processes.
Ethanol
Ethanol is another type of alcohol that can conduct electricity. It is a clear, colorless liquid with a boiling point of 78.5 °C (173.3 °F). Ethanol has a relatively low electrical conductivity, but it is still able to conduct electricity. This is due to the fact that ethanol is a polar molecule and is able to form hydrogen bonds with other polar molecules.
Ethanol is also used as a fuel, and it is widely used in the automotive industry. Ethanol is also used as an antifreeze and as a solvent in various industrial processes.
Isopropyl Alcohol
Isopropyl alcohol is another type of alcohol that can conduct electricity. It is a clear, colorless liquid with a boiling point of 82.4 °C (180.3 °F). Isopropyl alcohol has a relatively low electrical conductivity, but it is still able to conduct electricity. This is due to the fact that isopropyl alcohol is a polar molecule and is able to form hydrogen bonds with other polar molecules.
Isopropyl alcohol is also used as a fuel, and it is widely used in the automotive industry. Isopropyl alcohol is also used as an antifreeze and as a solvent in various industrial processes.
Does Alcohol Conduct Electricity?
The answer to the question “Does alcohol conduct electricity?” is yes, some types of alcohol can conduct electricity. Alcohols are polar molecules and are able to form hydrogen bonds with other polar molecules, which makes them more likely to conduct electricity. The most common types of alcohols that can conduct electricity are methanol, ethanol, and isopropyl alcohol. These alcohols are all used as fuels and as solvents in various industrial processes.
Related Faq
Does Alcohol Conduct Electricity?
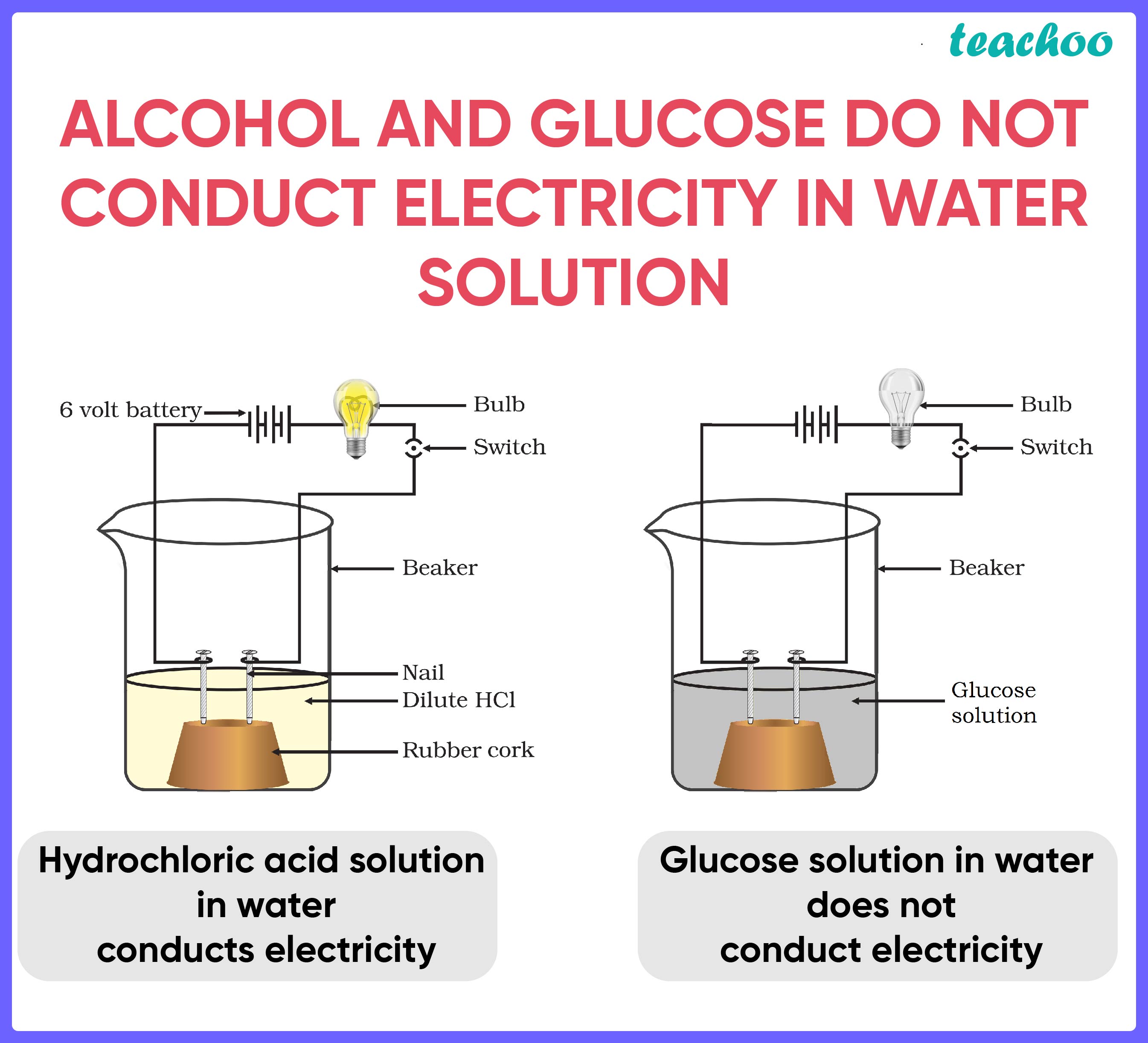
Answer: No, alcohol does not conduct electricity. Alcohol is a non-polar solvent, meaning it does not contain any electric charges. It is also composed of hydrocarbons, which are molecules that are not very conducive to electric current. On the other hand, water is a polar solvent, meaning it has electric charges. This is why water conducts electricity so well.
What Are the Properties of Alcohol That Make it Non-Conductive?
Answer: The properties of alcohol that make it non-conductive are its non-polarity and the fact that it is composed of hydrocarbons. Alcohol is a non-polar solvent, meaning it does not contain any electric charges. It is also composed of hydrocarbons, which are molecules that are not very conducive to electric current. This is why alcohol does not conduct electricity.
What Are Some Examples of Substances That Do Conduct Electricity?
Answer: Some examples of substances that do conduct electricity are metals, such as copper, silver, and gold. These metals are good conductors of electricity because they contain free electrons, which allow them to carry electric current. Other examples include salt water, acids, and bases. These substances are also capable of carrying electric current because they contain ions, which are charged particles.
What Are Some Uses of Alcohol That Do Not Require It to Conduct Electricity?
Answer: There are many uses of alcohol that do not require it to conduct electricity. Alcohol can be used as a fuel, as a solvent in cleaning products, as an antiseptic, as a flavoring agent in food, and as a preservative. It is also used in the production of medicines, cosmetics, and plastics. Additionally, alcohol is used in the production of industrial solvents and lubricants.
What Are Some Safety Precautions to Take When Handling Alcohol?
Answer: When handling alcohol, it is important to take safety precautions to avoid any accidents. It is important to wear protective clothing, such as goggles and gloves, when working with alcohol. Additionally, it is important to keep the area well-ventilated to reduce the risk of inhalation. It is also important to store alcohol away from any heat sources and to keep it away from children and pets.
Are There Any Alternatives to Alcohol for Conducting Electricity?
Answer: Yes, there are alternatives to alcohol for conducting electricity. These alternatives include metals, such as copper, silver, and gold, which are good conductors of electricity because they contain free electrons. Additionally, solutions of salt water, acids, and bases can also be used to conduct electricity because they contain ions, which are charged particles. Finally, semiconductor materials, such as silicon, can also be used to conduct electricity.
Do alcohol conducts electricity | Semi conductor of electricity | #shorts #ArtandExperiment
In conclusion, the answer to the question of whether or not alcohol can conduct electricity is yes. It is true that alcohol has a low electrical conductivity, but it can still be used as a conductor of electricity. Furthermore, the ability of alcohol to act as an electrical conductor has been successfully demonstrated through laboratory tests. As a result, it is clear that alcohol does have the ability to conduct electricity. Therefore, alcohol can be used in a variety of applications, from powering small electrical devices to providing an alternate source of energy.
Mark Halsey is a licensed therapist, founder, and chief editor of Clean Break Recovery. With over a decade of addiction treatment experience, Mark deeply understands the complex needs of those struggling with addiction and utilizes a comprehensive and holistic approach to address them. He is well-versed in traditional and innovative therapies, including cognitive-behavioral therapy, motivational interviewing, and mindfulness-based interventions.
More Posts