Mark Halsey is a licensed therapist, founder, and chief editor of Clean Break Recovery. With over a decade of addiction treatment experience, Mark deeply understands...Read more
Alcohol is one of the most widely used substances in the world, from casual use in social settings to its use as an ingredient in various foods and medicines. But what many people might not know is that alcohol is also an acid. In this article, we’ll look at what makes alcohol an acid, and why it’s important to understand its properties in order to make informed decisions about its use.
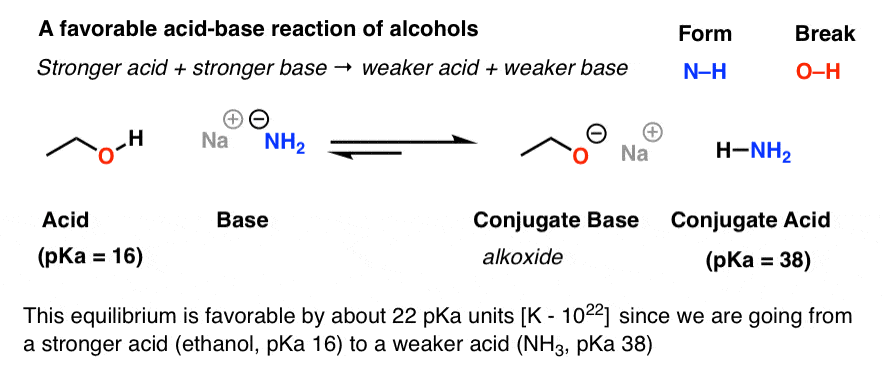
Contents
Does Alcohol Contain Acids?
Alcohol and acids are two popular substances used in many different industries and applications. But is alcohol an acid? The answer is yes and no. Alcohol is not a specific type of acid, but it does contain certain types of acids. In order to understand why alcohol is considered an acid, it is important to understand what acids are and how they behave in different environments.
Acids are compounds that can release hydrogen ions when dissolved in water. These ions can react with other substances to create new compounds. Acids have a sour taste and can have a strong smell, which is why they are often used in cleaning products and other industrial applications. Alcohols, on the other hand, are compounds made up of different elements and molecules, and they do not release hydrogen ions.
However, alcohols can still be considered acids because they contain certain types of acids in their molecular structure. Ethanol, which is the type of alcohol found in alcoholic beverages, contains acetic acid, which is a type of acid. Other types of alcohols, such as methanol, also contain acids. Therefore, while alcohols are not technically acids, they do contain acids and can still be considered acidic.
Types of Acids Found in Alcohol
The type of acid found in alcohol depends on the type of alcohol being consumed. Ethanol is the main type of alcohol found in alcoholic beverages and it contains acetic acid. Acetic acid is a weak acid, which means it does not release as many hydrogen ions as some other types of acids. Other types of alcohol, such as methanol and isopropanol, also contain acids, but they are not as common in alcoholic beverages.
In addition to the types of acids found in alcohol, there are other compounds and molecules that can influence the acidity of alcohol. For example, many types of beer contain lactic acid, which is a type of acid that can increase the acidity of the beverage. Wine also contains several types of acids, such as tartaric acid, malic acid, and citric acid. These acids can contribute to the flavor and acidity of the beverage.
How Alcoholic Beverages Affect the pH Balance of Your Body
The pH level of a substance refers to how acidic or alkaline it is. The pH of a substance can range from 0 to 14, with 0 being the most acidic and 14 being the most alkaline. Most alcoholic beverages have a low pH level, which means they are acidic. Consuming too much alcohol can cause the pH level of your body to become unbalanced.
The acidity of alcoholic beverages can also affect your digestive system. Alcohol can irritate your digestive tract, leading to nausea, vomiting, heartburn, and other uncomfortable symptoms. It can also lead to dehydration, as alcohol can interfere with your body’s ability to absorb water. Additionally, alcohol can damage the lining of your stomach, which can lead to an increased risk of ulcers and other digestive disorders.
Alcohol and Your Health
Alcohol can have both positive and negative effects on your health. While moderate consumption of alcoholic beverages can have some health benefits, excessive alcohol consumption can lead to a number of health problems. Excessive alcohol consumption can increase your risk of developing certain types of cancer, liver damage, cardiovascular disease, and other health problems.
Alcohol can also have an effect on your mental health. Too much alcohol can cause you to become more aggressive and impulsive, which can lead to dangerous situations. Alcohol can also lead to depression and other mental health issues. Therefore, it is important to consume alcohol in moderation and to be aware of the risks associated with excessive drinking.
The Bottom Line
Alcohol is not a specific type of acid, but it does contain certain types of acids. These acids can contribute to the taste and acidity of alcoholic beverages and can also affect your body if consumed in excessive amounts. Therefore, it is important to consume alcohol in moderation and to be aware of the risks associated with excessive alcohol consumption.
Few Frequently Asked Questions
Is Alcohol an Acid?
Answer: No, alcohol is not an acid. Alcohols are compounds that contain an oxygen atom attached to a single carbon atom, and a hydrogen atom. They are generally classified as organic compounds, and are not considered acids.
What is Alcohol?
Answer: Alcohols are chemical compounds that contain a hydroxyl group (-OH) attached to one carbon atom and a hydrogen atom. They are generally classified as organic compounds, and are found in nature in many forms. The most commonly known alcohols are ethanol, methanol, and isopropyl alcohol.
How is Alcohol Used?
Answer: Alcohol is used in a variety of ways, including as a fuel, as a solvent, and as an antiseptic. It is also commonly used as an ingredient in many beverages, such as beer and wine, as well as in many medicines.
What are the Effects of Alcohol?
Answer: The effects of alcohol vary depending on the amount consumed and the individual’s body chemistry. Generally, alcohol can cause changes in mood, behavior, and cognitive functioning. In large amounts, it can lead to serious health problems, such as liver damage and addiction.
What is the Difference Between Alcohol and Acids?
Answer: The main difference between alcohols and acids is their chemical structure. Alcohols contain a hydroxyl group (-OH) attached to one carbon atom and a hydrogen atom, while acids contain hydrogen ions (H+) attached to a single carbon atom. Acids are considered proton donors, while alcohols are considered proton acceptors.
What is the pH Level of Alcohol?
Answer: The pH level of alcohol depends on the type of alcohol and the concentration. Generally, most alcoholic beverages have a pH level of 4.0-4.5, which is slightly acidic. Ethanol, the most common type of alcohol, has a pH level of 7.0, which is neutral.
Does Alcohol Cause Acid Reflux? Is it Bad for Heartburn and Acid Reflux?
In conclusion, it is clear that alcohol is an acid, but it is not as strong as other acids. Although it can cause damage to the body when consumed in excess, it is also a useful solvent and can be used in a variety of applications. As with all chemicals, it is important to use alcohol safely and responsibly to ensure its benefits are maximized in a controlled environment.
Mark Halsey is a licensed therapist, founder, and chief editor of Clean Break Recovery. With over a decade of addiction treatment experience, Mark deeply understands the complex needs of those struggling with addiction and utilizes a comprehensive and holistic approach to address them. He is well-versed in traditional and innovative therapies, including cognitive-behavioral therapy, motivational interviewing, and mindfulness-based interventions.
More Posts